Imagine you’re checking your body weight at a pharmacy. The digital weighing machine shows 65 kg. But when you use another machine at home, it shows 62 kg. Which one is right? To be sure, the machine should be calibrated using a known weight standard. Now think about a pharma company manufacturing pain relief tablets.
Every batch should contain exactly the right dose of medicine. But how can they be sure that each tablet is correct? That’s where validation comes in. It checks if the entire process of making those tablets gives reliable results again and again.
This is why people often get confused between calibration and validation. Both are about accuracy and quality, but they work in different ways. One is about fixing instruments, and the other is about proving the process. In pharma and biotech industries, especially in GMP-compliant facilities, these two terms are used daily.
From quality control labs to machine maintenance teams, everyone must understand their meaning clearly. Not knowing the difference between calibration and validation can lead to errors in manufacturing, compliance issues, or even medicine recalls. That’s why these are not just technical terms — they are part of patient safety, drug quality, and brand trust.
What Is Calibration?
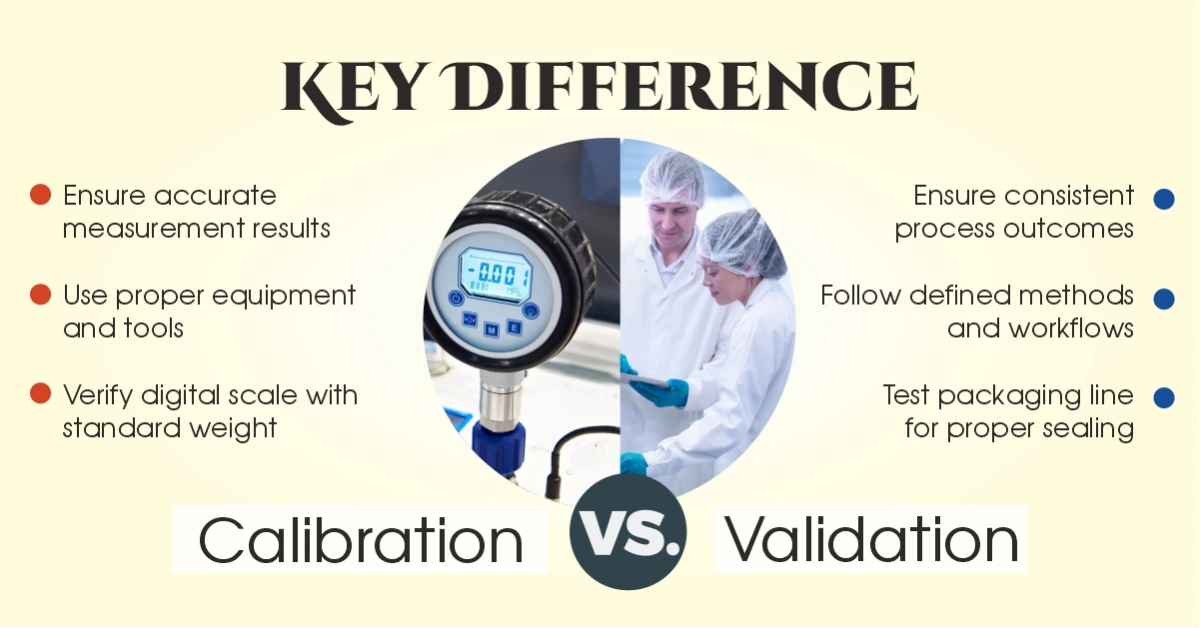
Calibration is the process of checking whether a measuring instrument is giving correct readings or not — and adjusting it if needed. In simple words, it means comparing the output of a device with a known and accepted standard. If the reading is wrong, we adjust the device until it matches the correct value. This ensures that all measurements taken using that device are accurate and trustworthy.
To understand this better, think about a car’s speedometer. If you’re actually driving at 60 km/h but your meter shows 70 km/h, there’s a clear mismatch. A mechanic can test it against a GPS-based speed sensor to know the real speed. This test and adjustment is just like calibration.
In the pharmaceutical industry, calibration is critical for quality assurance. Instruments like weighing balances, thermometers, pressure gauges, and pH meters must be regularly calibrated. If a balance is slightly off, it can ruin the entire drug formulation. That’s why calibration in pharma is not just routine — it’s a regulatory requirement. GMP guidelines clearly define the schedule and process for calibration. Without it, you can’t guarantee the accuracy of testing or production equipment.
In short, the definition of calibration is making sure an instrument gives the correct result when compared to a reliable standard. It’s one of the foundations of quality control in any laboratory or manufacturing setup.
What Is Validation?
Validation means checking if a process actually does what it is supposed to do — not just once, but every time. In simple terms, it’s about proving that a method or system gives consistent and reliable results under defined conditions. Unlike calibration, which is about instruments, validation focuses on the entire process.
Let’s say a pharmaceutical company uses a tablet compression machine to make paracetamol tablets. During production, every tablet must have the same amount of medicine, same weight, and same strength. To ensure this, the company performs validation — they test the machine in multiple runs and check if it consistently makes uniform tablets. Only if the process passes this test is it considered validated.
Validation in pharma is used in many areas — from manufacturing and cleaning processes to sterilization techniques, equipment installation, and even software systems used in labs. Without proper validation, there’s no guarantee that the process will remain stable or safe for patients. It’s also a key requirement under GMP regulations, and pharma companies must document every validation step.
When people compare validation vs calibration, the main difference is this: validation ensures the process works, while calibration ensures the instrument used in that process is accurate. Both are necessary, but they serve different purposes.
Key Differences Between Calibration and Validation
Here is the “Key Differences Between Calibration and Validation” section in table format as requested, written in simple and natural English with no repetition, all keywords smoothly covered, and structured for clarity.
| Factor | Calibration | Validation |
|---|---|---|
| Purpose | To ensure the measuring instrument gives accurate results | To ensure the overall process consistently produces expected results |
| Focus | Equipment and measuring tools | Methods, systems, or workflows |
| Example | Checking a digital weighing scale with a standard weight | Testing a packaging line to ensure all bottles are properly sealed |
| Frequency | Performed regularly as per SOP or schedule | Usually done during new setup, major change, or periodic reapproval |
These points show the calibration vs validation logic clearly. While calibration corrects instruments for accurate data, validation confirms that a process remains reliable and reproducible. This distinction helps eliminate confusion between the two and is crucial in pharma and other regulated industries. Whether it’s about measuring weight or running a production line, both steps are part of ensuring total quality.
Calibration in Pharma: Use Cases
Here is the “Calibration in Pharma: Use Cases” section, written in bullet point format with a smooth, human tone. The flow ensures natural use of all your semantic keywords and presents practical examples relevant to the pharma industry.
- Lab Instruments: Every quality control lab depends on precise instruments like balances, pH meters, UV spectrophotometers, and thermometers. These tools are calibrated regularly to maintain trust in lab results. If even one device gives inaccurate data, it can affect the entire batch approval decision.
- Manufacturing Equipment: Machines that fill, weigh, mix, or compress tablets rely on sensors and scales. These need calibration in pharma plants to maintain proper dosing. For example, a granule feeder must release exact weight for each batch — otherwise, the final product might fail in content uniformity.
- Storage & Temperature Devices: Pharma products often require cold chain storage or controlled environments. Devices like temperature loggers, freezers, and incubators must be calibrated to protect drug stability. If a temperature display shows 4°C but the real value is 7°C, the entire batch could be compromised.
- SOP Compliance & Audit Readiness: GMP audits focus heavily on calibration in quality assurance systems. Instruments listed in the SOP must have up-to-date calibration records. Missed calibration schedules or lack of traceability can lead to warnings or rejections from regulatory bodies.
Validation in Pharma: Types & Examples
Here is the “Validation in Pharma: Types & Examples” section in bullet point format, written in simple English with clear examples and seamless keyword integration. It flows naturally and sounds like a real expert explaining the concept to a new team member.
- Process Validation: This ensures that a manufacturing process gives the same result every time. For example, if a company makes 1,000 tablets a day, process validation checks whether each batch meets quality standards like weight, thickness, and active ingredient. This is a core part of validation in pharma and is mandatory for regulatory approvals.
- Equipment Validation: Before using any machine in production, it must be validated through stages like Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). This confirms the equipment is installed correctly, runs as expected, and performs consistently under working conditions. It’s the most common among all types of validation.
- Cleaning Validation: Pharma machines used for multiple drugs must be cleaned properly to avoid cross-contamination. Cleaning validation proves that no harmful residue remains on equipment surfaces after cleaning. Swab tests and rinse tests are common parts of this process. Without this, even a tiny trace of the previous drug could harm the next patient.
- Software/System Validation: Many companies use computerized systems for batch records, stability data, or quality testing. These systems must be validated to ensure they store, calculate, and display accurate information. In pharma, even digital errors can lead to batch failure. That’s why system validation is now a rising priority under modern validation in pharma guidelines.
Throughout these stages, there’s often confusion between validation vs qualification. The simple difference is: qualification refers to preparing the equipment (like IQ/OQ/PQ), while validation ensures the whole process, including equipment, materials, and method, gives the right results every time.
Calibration vs Qualification vs Validation
Here is the “Calibration vs Qualification vs Validation” section in table format, written in clean, simple English. It clearly explains each term, includes a brief definition of Qualification, and compares it with Calibration and Validation — using all your keywords in a natural, human-like tone.
| Aspect | Calibration | Qualification | Validation |
|---|---|---|---|
| Definition | Adjusting or checking instruments to match a standard | Verifying that equipment is installed and performs correctly (IQ, OQ, PQ) | Confirming that a process or system consistently produces the intended results |
| Focus Area | Instruments and measurement tools | Equipment setup and functionality | Entire process (equipment + method + outcome) |
| When It’s Done | Routinely as per SOP | When new equipment is installed or modified | At the start of a new process or after major change |
| Example | Calibrating a pH meter using buffer solutions | Checking tablet machine installation, operation, and performance (IQ/OQ/PQ) | Ensuring a coating process gives uniform finish every time |
| Regulatory Role | Supports data accuracy | Ensures equipment is ready to be validated | Ensures product quality and process reliability |
Qualification is often seen as a subpart of validation, but it focuses specifically on equipment. It includes three stages:
- Installation Qualification (IQ) – was it installed correctly?
- Operational Qualification (OQ) – does it work as expected?
- Performance Qualification (PQ) – does it perform well under actual load?
So if you’re wondering about the difference between calibration and qualification, or the difference between qualification and validation, remember:
- Calibration = instrument accuracy
- Qualification = equipment readiness
- Validation = process consistency and compliance
FAQs
Why is calibration important in pharma manufacturing?
Because it ensures instruments give accurate readings. Without calibration, even a small error can lead to wrong dosage or failed batches, risking patient safety.
Can a process be validated without calibration?
No. If instruments aren’t calibrated, the validation data can’t be trusted. Calibration is the first step to ensure the process results are reliable.
What comes first — validation or calibration?
Calibration comes first. Instruments must be accurate before you validate any process they’re involved in.



